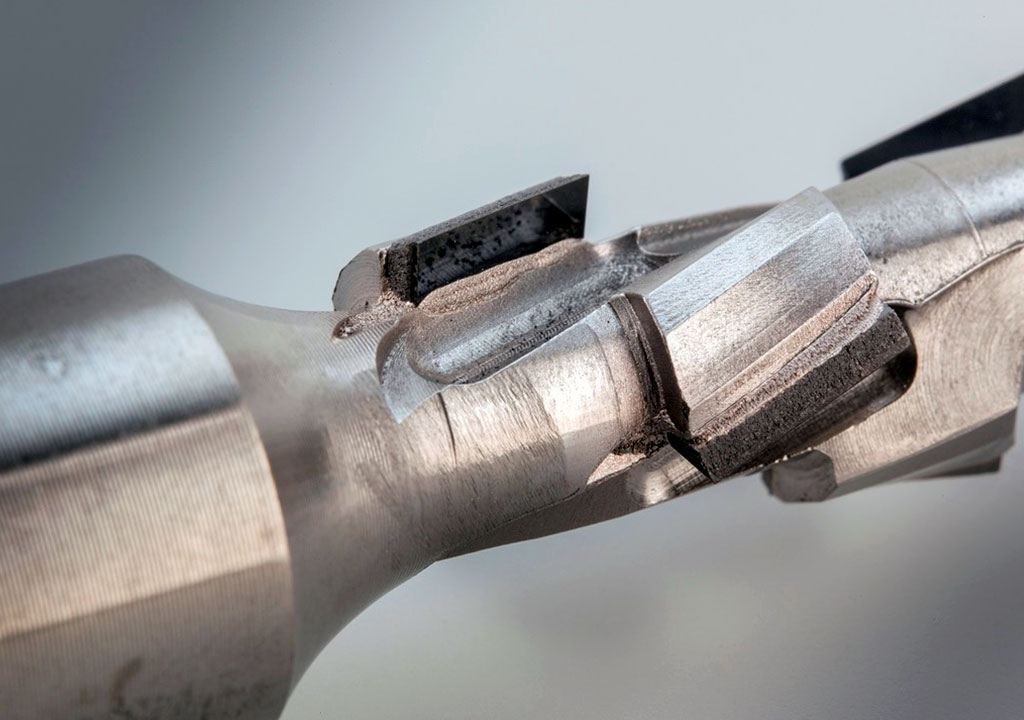
Brazing Diamond
Researchers have discovered that by adding Ti to brazing alloys, stable carbides are formed which wet and allow the brazing of diamond to many materials.
These alloys are knowns as active brazing filler metals: Alloy systems including Cu-Ag-Ti, are use brazing filler metals for joining diamonds to metals, and ceramics. Check our list @:
The most important factor to consider when brazing diamonds is the graphitization. Once the diamond has undergone this transformation, it losses its desired mechanical properties. When graphitization occurs is a function of type of diamond, oxygen level and temperature. For this reason low temperature metals such Indium can be added in the composition.
“The most important factor to consider when brazing diamonds is the graphitization. Once the diamond has undergone this transformation, it losses its desired mechanical properties.”
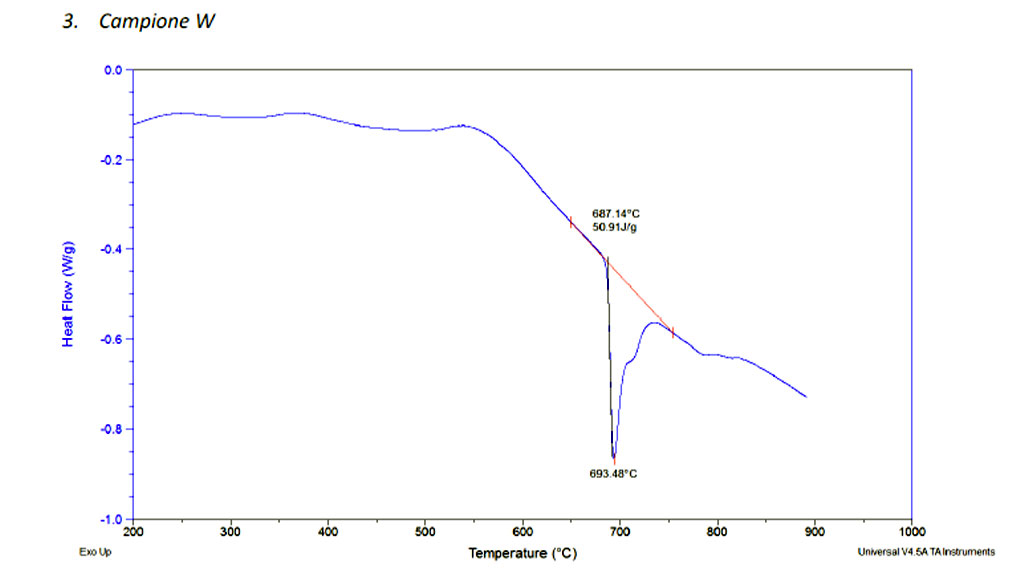
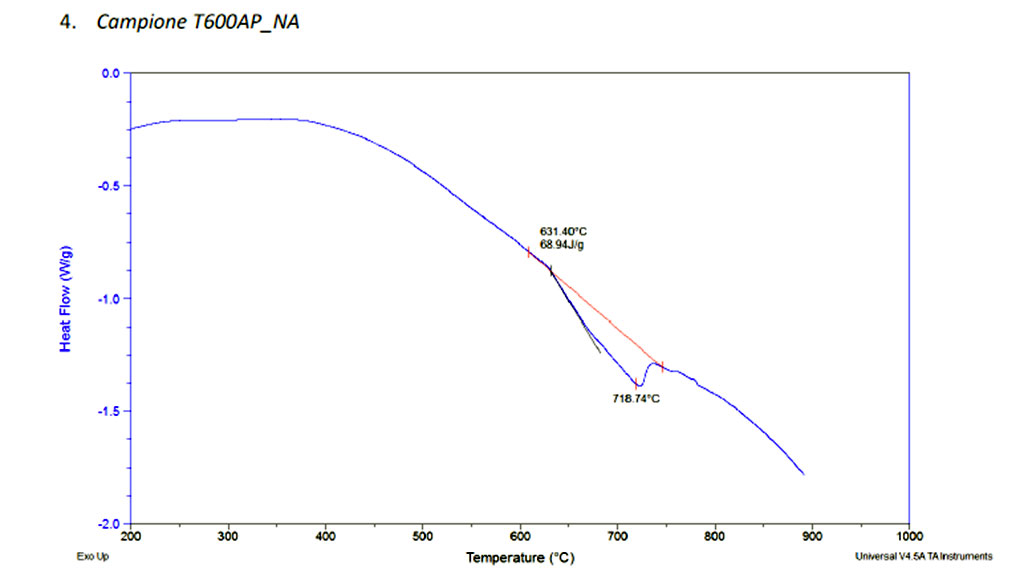
Our goal to reduce the temperature lead us to the development of different alloys such T600AP and T600AWP, those alloys were especially project for diamond bonding.
Chemical Composition ( % )
| A.V. |
Ag Min. Max. |
Cu Min. Max. |
Ti Min. Max. |
In Min. Max. |
|
|---|---|---|---|---|---|
| T600AMW11XS |
39 41 |
39 41 |
9 11 |
8 11 |
Work. temp: 700-750°C Melt. range: 695°-700°C |
Chemical Composition ( % )
| A.V. |
Ag Min. Max. |
Cu Min. Max. |
Ti Min. Max. |
In Min. Max. |
Other Min. Max. |
|---|---|---|---|---|---|
| T600A |
57 60 |
25 29 |
0.8 1.5 |
11 13 |
Work. temp: 720-750°C Melt.range: 715°-740°C |
Special Binder XS is design to evaporate and glue the join before the brazing cycle (usually in vacuum furnace). 15-20 min at 100°C-125°C depending on the dimension and complexity of the join. The binder will also evaporate at room temperature in few hours.